It is carried out when any item is going to be manufactured by using a new components or in a new facility. Also called premarket validation, prospective validation is often completed right before commencing regime production.
Discover the value of machines style in cleansing validation And the way ValGenesis Process Supervisor increases sampling designs and makes sure compliance. Peter Liang ValGenesis provides integrated and clever solutions that aid the digital transformation of your life sciences industry.
Meeting regulatory demands is paramount With regards to process validation. So as to make sure the security and efficacy of pharmaceutical products, regulatory bodies like the FDA as well as EMA have established guidelines that has to be adopted. Let us check out these guidelines in more depth:
PAT presents a prosperity of up-to-date info, letting stakeholders to produce strategic conclusions as opposed to counting on blind guesses. This hurries up determination-producing, enabling brand names to catch top quality challenges early and launch merchandise more quickly than their competitors.
In this particular stage, the process is built and documented intimately. The critical process parameters as well as corresponding functioning ranges are identified.
In this particular stage, the process layout is assessed to conclude if the process has the capacity to fulfill determined manufacturing requirements. With this phase all output processes and producing products is proofed to substantiate quality and output abilities.
Also, process validation plays a crucial position in making sure item basic safety. It can help identify opportunity dangers and website deviations that can compromise the protection of the top solutions. By addressing these dangers and deviations, providers can make certain that their merchandise are Risk-free for individuals to utilize.
By validating the manufacturing process, firms can lower the chance of defects, faults, and deviations that can influence solution quality, security, and efficacy. This not just assures buyer fulfillment but in addition assists retain regulatory compliance and stop pricey recollects.
Other Back again while in the nineteen sixties, pharmaceutical items have only been examined just after generation. If the ultimate solution achieved the expectations, It will be cleared for patient use. This technique remained unchanged till the seventies each time a number of incidents shook the industry.
Process validation also contributes to steady enhancement initiatives in an organization. By analyzing process data and identifying spots for advancement, providers can boost their producing processes, leading to improved effectiveness, lessened waste, and improved In general functionality.
The validation report arranges a particular feature of report formats considering the fact that distinct data must be taken Take note of in arranging to deliver an outstanding acceptance report. Every acceptance report’s material should summarize all that’s predicted from it by evaluators and reviewers. It's also possible to check Report Sample Doc.
Excellent groups must know which characteristics to monitor to make click here sure the manufacturing process operates easily. That may be why many corporations switch to facts analytics to pinpoint the parameters that effect output one of the most.
In preceding report we comprehended precisely what is pharmaceutical validation examine and scope (phase)of validation. We also saw the history
Such a process validation happens for the duration of the development stage prior to product or service internet marketing to shoppers. The primary objective is to make certain the generation design and style fulfills all vital requirements.
 Barry Watson Then & Now!
Barry Watson Then & Now!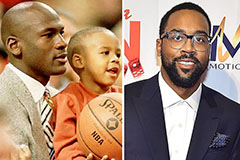 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!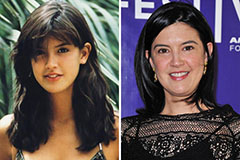 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Bo Derek Then & Now!
Bo Derek Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now!